Foraging ecology of the amphibious mudskipper Periophthalmus chrysospilos (Gobiiformes: Gobiidae)
- Published
- Accepted
- Received
- Academic Editor
- Khor Waiho
- Subject Areas
- Aquaculture, Fisheries and Fish Science, Ecology, Zoology, Freshwater Biology
- Keywords
- Carnivorous, Specific feeder, Food composition, Mudskipper
- Copyright
- © 2021 Dinh et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Foraging ecology of the amphibious mudskipper Periophthalmus chrysospilos (Gobiiformes: Gobiidae) PeerJ 9:e12582 https://doi.org/10.7717/peerj.12582
Abstract
The food composition and feeding ecology of fishes living in the intertidal zone play an essential role in understanding the energetic connectivity between terrestrial and aquatic systems. Periophthalmus chrysospilos is an amphibious fish species occurring in the intertidal zone, but data on its diet and foraging ecology is still poorly known. This study on Ps. chrysospilos was carried out from April 2020 to March 2021 at four sites within the Mekong Delta estuary to define the influence of spatio-temporal factors on the diet of this species. The diet composition and relative gut lengths (RGLs) of Ps. chrysospilos were analysed in relation to four parameters—sex, size, site, and season. A total of 1,031 individuals were collected, and their digestive tract lengths were used to calculate the RGL. The digestive tracts of only 546 individuals were with food items (approximately 1:1 of empty vs full digestive tract) and were subsequently used for further analyses. The ranges in total length and weight in both adult and juvenile individuals were 3.4–10.6 cm and 0.38–14.13 g, respectively. The RGL values varied with season, fish size and site, but was always lower than 1, indicating a predominantly carnivorous diet. The variability of food items found within the digestive tracts demonstrated its adaptability in pursuing prey items within the limits of the littoral zone, and its importance as a conduit of terrestrial-marine connectivity. This species is characterised as an opportunistic mesopredator feeding primarily on Acetes spp., Uca spp., Dolichoderus sp., and rarely on Polychaeta and Actinopterygii. Other items found within the digestive tract are Mollusca, and detritus. The diet composition of Ps. chrysospilos did not vary with season and size, but changed with sex and site parameters. Uca spp. contributed to the sexual variation in dietary component, whereas Mollusca, Uca spp., Dolichoderus sp. and detritus, were drivers for spatial variation in the dietary component. The research provides fundamental information on diet composition and feeding strategy, as well as contributes towards knowledge on foraging ecology and resource use by intertidal animal communities.
Introduction
Fishes, occupying all trophic levels within aquatic environments, are integral components to elucidate aquatic trophic ecology (Wootton, 1996; Blaber, 2000). In intertidal systems where terrestrial elements factor significantly in trophic ecology, studies traditionally focus on the impact of the environment on the foraging strategy of fishes (Ravi, 2013; Tran, Hoang & Dinh, 2019; Dinh et al., 2020b). During the ebb tide, the landscape of intertidal areas changes drastically. Mudskippers are some of the few fishes that have adapted to aerial exposure, and remain on these exposed littoral areas where they actively forage (Murdy & Jaafar, 2017). There are forty-three species of mudskippers, with varying sensitivities to aerial exposure and foraging behaviour (Jaafar & Murdy, 2017). Some species, such as those from the genus Boleophthalmus, are less tolerant to desiccation, stay close to the waterline, and graze the substrate surface for diatoms (Ravi, 2013; Dinh, 2015). Others, such as species of Periophthalmodon, are carnivorous and are known to traverse upriver to areas unaffected by tidal cycles (Dinh, 2018a, 2018b; Dinh, Tran & Nguyen, 2018; Dinh et al., 2020b).
Of the ten genera of mudskippers, the genus Periophthalmus is most speciose with 19 species and considered to be most tolerant to aerial exposure (Jaafar & Murdy, 2017; Dinh et al., 2021a). These fishes are extremely active during the ebb tide. Individuals are observed to forage throughout the mangrove zones, defend their territories, pursue mates, and maintain burrows; the latter oft in pairs (Polgar & Crosa, 2009; Murdy & Jaafar, 2017). Periophthalmus (‘Ps.’ hereafter) species are omnivorous and opportunistic foragers, and these are reflected in their morphology. Their teeth are typically caniniform, thick basally and recurved at the tips (Sponder & Lauder, 1981), while their gill rakers are short, knob-like projections that are widely separated (Mazlan, Masitah & Mahani, 2006). However, plant material is often found in the digestive tracts although there is still no consensus on its role in their diet. Some studies for example, report it as a principal diet component (Bob-Manuel, 2011; Udoh et al., 2013), while others consider it incidental intake (Clayton, 2017) or an alternate food source during winter months (Mhaisen & Al-Maliki, 1996). Species of Periophthalmus are known to be visual (Clayton, 1993; Kutschera, Burghagen & Ewert, 2008) and tactile hunters (Colombini et al., 1996; Somerfield, Gee & Aryuthaka, 1998), possessing well-developed olfactory epithelia (Kuciel, Żuwała & Jakubowski, 2011; Kim, Yun & Park, 2019) important receptors of olfactory cues during foraging bouts.
The prominence of these mesopredators within the intertidal zone, makes species of Periophthalmus excellent candidates to understand the energetic connectivity between terrestrial and aquatic systems. Yet, current information on the effects of spatio-temporal factors on the diet and foraging ecology of these fishes remain scarce (Ravi, 2013; Tran, Hoang & Dinh, 2019; Dinh et al., 2020b). We study the diet composition of an obligate intertidal species, Periophthalmus chrysospilos Bleeker 1853 (Gobiidae: Oxudercinae). Unlike all other species within this genus, Ps. chrysospilos forage in small groups of 20–40 individuals along the ebbing waterline (Milward, 1974; Polgar & Crosa, 2009). Through the analyses of food items within digestive tracts, we aim to define the food composition and investigate if the diet of this species changes with size, sex, site, and season. Our study on the diet composition of this species contributes towards knowledge on foraging ecology and resource use by intertidal animal communities.
Materials and Methods
Sampling sites
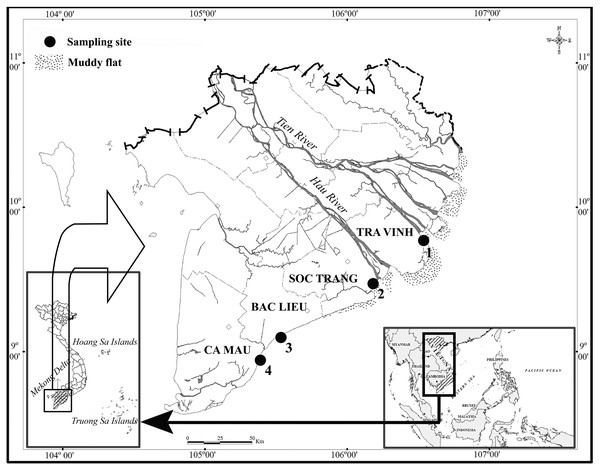
Specimens were collected from April 2020 to March 2021 at four locations along the Hau river estuary in Vietnam (Fig. 1): Duyen Hai-Tra Vinh (DHTV; 9°41′18.6″N 106°30′35.8″E), Tran De-Soc Trang (TDST; 9°29′26.8″N 106°11′58.5″E), Dong Hai-Bac Lieu (DHBL; 9°06′03.2″N 105°29′49.1″E); and Dam Doi-Ca Mau (DDCM; 8°58′17.5″N 105°22′51.8″E). There is little fluctuation in temperature at the sites between wet (June–December) and dry (January–May) seasons; the temperature remains at approximately 27 °C. Conversely, precipitation measures varied significantly—a monthly average of 20 mm in the dry season and 400 mm in the wet season (Le et al., 2006). The pH was 7.6–7.9 and varied with sites but not seasons, whereas the salinity was 12.3–23.5% and varied with season but not site (Dinh et al., 2021b).
Figure 1: Four sites of this study indicated within the map in the Mekong Delta.
Source credit: Dinh (2017) © John Wiley and Sons.Vegetation typical at these sites include Acanthus ebracteatus Vahl., Avicennia marina (Forssk.) Vierh., Bruguiera gymnorrhiza (L.), Nypa fruticans Wurmb., Savigny Sonneratia caseolaris (L.) A. Engl., and Rhizophora apiculata Blume. The dominant plant species at DHTV, TDST and DHBL were Sonneratia caseolaris, Avicennia marina and Bruguiera gymnorrhiza (L.) Savigny., respectively. At DDCM, Avicennia marina and Bruguiera gymnorrhiza (L.) Savigny. were equally dominant (Dinh et al., 2021b).
Sample collection
Fishes were randomly collected ∼4 hours during the ebb tide by hand in an area of 120 square metres (6 m × 15 m) on the mudflat at night for five consecutive days per month. Fishes were identified following Murdy & Jaafar (2017) and sexed. The genital papillae of females are bulbous, pinkish, and equally broad at the base and tip, whereas those for males are slender and whitish, broad at the base and tapers towards the tip (Dinh et al., 2020a). Fishes captured were immediately euthanised in a solution of tricaine methanesulfonate (MS222) before being transferred into a solution of 5% buffered formalin and transported to the laboratory. In the laboratory, specimens were soaked in water for 2 hours before analyses. The total length (TL, nearest to 0.1 cm) and weight (W, nearest to 0.01 g) of each specimen was recorded before dissection and analyses of content of the digestive tract. The length at first maturity (Lm) was used to divide the fishes into two groups: immature group if TL < Lm and mature group if TL ≥ Lm. Individual of females and males from DHTV with total lengths of 7.3 cm and 6.9 cm or below were considered immature. Lm values of females and males from TDST were 6.4 cm and 6.6 cm; 7.0 cm and 6.2 cm from DHBL; and 6.8 cm and 8.6 cm from DDCM (Q. M. Dinh, 2021, Unpublished data).
The use of fishes in the present study was assessed and approved by The Council for Science and Education, School of Education, Can Tho University (Animal Welfare Assessment number: BQ2020-03/KSP). Altogether, 1,031 specimens were used for RGL and 546 specimens for diet composition analyses.
Relative gut length
The digestive tract of each specimen was carefully removed and measured (nearest 0.1 cm) to obtain the relative gut length (RGL = total length of the gut/the total length of fish). The association of the length of digestive tract to the length of the fish has been used as an indicator for feeding guild (see Al-Hussaini (1947), Kapoor, Smit & Verighina (1976), Drewe et al. (2004)). Typically, RGL value greater than 3 signifies that the species is herbivorous, RGL value between 1 and 3 signifies omnivorous fishes, and RGL value below 1 signifies carnivorous fishes.
Diet composition
Contents of each digestive tract were analysed under the stereomicroscope (Motic DM-143-FBGG) and identified to the lowest possible taxonomic level following Dill (2002) and Nguyen et al. (2013). The occurrence of each food item was calculated by the following equation: %Oi = 100 × Oi/N (Hynes, 1950), where Oi is the number of fishes consuming prey i and N is the total number of fishes examined. The weight of each food item was calculated by the following equation: %Wi = 100 × Wi/Wtotal (Hyslop, 1980), where, Wi is weight of prey i, Wtotal is total weight of all prey individuals. Biovolume of prey was calculated by the following equation: %Vi = (100 × Oi × Wi)/Σ(Oi × Wi), where Vi, Oi and Wi are the percentage of biovolume, occurrence and weight of prey i respectively. The diet composition was analysed against sex, size, site, and season to understand the interactions of these parameters to food acquisition (Natarajan & Jhingran, 1961; Hyslop, 1980; Clayton, 2017; Dinh et al., 2020b).
The modified graphical method of Costello (1990) was used to visualise the diet composition of Ps. chrysospilos (Amundsen, Gabler & Staldvik, 1996). Diet components that are highly abundant and essential appear at the upper right quadrant of the graph, while less abundant but essential components appear at the lower right quadrant of the graph; less essential components that are abundant or scarce appear on the left upper and lower quadrants respectively (Adámek, Andreji & Gallardo, 2007).
Data analyses
PRIMER v.6.1.11 (Clarke & Gorley, 2006) with PERMANOVA+ v.1.0.1 add-on package (Anderson, Gorley & Clarke, 2008) was used to test if variation in diet can be attributed to the sex and size of the fish, or to site and season of catch (Baeck, Yoon & Park, 2013). If variations were detected, nonparametric tests would be applied to identify the driver for the differences (Dinh et al., 2017). The Mann-Whitney U test was used to test for changes in diet composition between factor pairs: sex (male and female individuals); size (immature and mature were determined basing on the Lm value as mentioned in the section on fish collection); and season (dry and wet). If diet composition varied for more than two factors, the Kruskal-Wallis H test was applied to identify the component driver.
T-test was applied to test the variations of RGL between fish sex, sizes, and season. One-way ANOVA was used to test the variation of RGL between sites. The effect of interactions between factors such as: gender × size; gender × season; gender × site; size × season; size × site; season × site; gender × size × season; gender × size × site; gender × season × site; size × season × site; and gender × size × season × site on RGL were quantified using General Linear Model. The significant level was p < 0.05 in all tests. To decrease the likelihood of Type I error of all test, the Benjamini–Hochberg procedure was applied (Benjamini & Hochberg, 1995; McDonald, 2014).
Results
A total of 1,031 Ps. chrysospilos specimens were captured from four sites (508 females and 523 males) over a span of 1 year, from April 2020 to March 2021,. The highest quantity of fishes was collected from Dong Hai, Bac Lieu (303) while the lowest was from Dam Doi, Ca Mau (229). The number of Ps. chrysospilos recovered monthly from each site ranged from 10 to 42 fishes, including those with full and empty digestive tracts. The total length and weight of Ps. chrysospilos ranged 3.4–10.6 cm and 0.38–14.13 g, respectively. The most specimens of Ps. chrysospilos, at 116 individuals, was collected in July while the fewest specimens collected, at 64 specimens, was collected in October. Of these, the digestive tracts of 546 individuals were found with food, while the digestive tracts of the remaining 485 individuals were empty (see Table 1). Overall the ratio of fishes with empty vs full digestive tracts was approximating 1:1. Dong Hai, Bac Lieu was the site with the highest occurrence of individuals (164 individuals) with empty digestive tracts. The month with the least and most fishes with empty digestive tracts was July (56 individuals) and October (27 individuals) respectively.
| Year | Month | Duyen Hai, Tra Vinh | Tran De, Soc Trang | Dong Hai, Bac Lieu | Dam Doi, Ca Mau | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total specimens | Empty DT | Total specimens | Empty DT | Total specimens | Empty DT | Total specimens | Empty DT | ||||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | ||
| 2020 | April | 20 | 8 | 8 | 3 | 6 | 14 | 1 | 6 | 9 | 21 | 6 | 13 | 7 | 8 | 4 | 6 |
| 2020 | May | 10 | 7 | 4 | 2 | 12 | 12 | 6 | 5 | 12 | 8 | 5 | 4 | 11 | 6 | 5 | 4 |
| 2020 | June | 7 | 17 | 3 | 0 | 5 | 28 | 2 | 9 | 17 | 17 | 6 | 8 | 13 | 12 | 12 | 11 |
| 2020 | July | 14 | 7 | 6 | 2 | 19 | 9 | 4 | 4 | 12 | 18 | 10 | 13 | 16 | 15 | 7 | 10 |
| 2020 | August | 7 | 14 | 2 | 5 | 5 | 5 | 2 | 1 | 17 | 11 | 4 | 3 | 15 | 6 | 8 | 3 |
| 2020 | September | 13 | 6 | 6 | 2 | 13 | 4 | 6 | 4 | 26 | 16 | 22 | 12 | 11 | 9 | 3 | 1 |
| 2020 | October | 3 | 14 | 2 | 2 | 11 | 9 | 5 | 2 | 7 | 9 | 6 | 6 | 6 | 5 | 3 | 1 |
| 2020 | November | 6 | 11 | 3 | 2 | 13 | 7 | 7 | 3 | 14 | 5 | 8 | 3 | 11 | 6 | 4 | 3 |
| 2020 | December | 7 | 12 | 6 | 8 | 8 | 15 | 5 | 8 | 8 | 16 | 3 | 7 | 10 | 6 | 3 | 0 |
| 2021 | January | 9 | 9 | 7 | 6 | 10 | 16 | 4 | 7 | 5 | 15 | 2 | 2 | 11 | 6 | 2 | 1 |
| 2021 | February | 11 | 6 | 10 | 6 | 9 | 12 | 2 | 2 | 12 | 8 | 3 | 5 | 11 | 5 | 5 | 3 |
| 2021 | March | 13 | 8 | 8 | 4 | 9 | 9 | 6 | 6 | 7 | 13 | 7 | 6 | 15 | 8 | 5 | 3 |
| Total Specimens | 120 | 119 | 65 | 42 | 120 | 140 | 50 | 57 | 146 | 157 | 82 | 82 | 137 | 92 | 61 | 46 | |
Relative Gut Length (RGL)
The RGL values were significantly different between individuals of different sizes (t-test, t = −5.39, n = 1.031, df = 1.029, p < 0.001, CI95% [−0.07 to −0.03]); mature individuals exhibited higher RGL values when compared to immature individuals (0.56 ± 0.006 and 0.51 ± 0.007), respectively, (see Table 2). However, the RGL values were not significantly different between males and females (t = 0.45, n = 1.031, df = 1.029, p = 0.15, CI95% [−0.01 to 0.02]; see Table 2). When testing for temporal variations, RGL values were significantly different between seasons (t = −12.90, n = 1.031, df = 1.029, p < 0.001, CI95% [−0.13 to −0.10]). The RGL value was higher in the wet season than the dry season (0.59 ± 0.01 and 0.47 ± 0.01, respectively, see Table 2). During the study duration, RGL values were the highest from July to November 2020 (around 0.61 ± 0.01–0.65 ± 0.016), while the lowest value was observed in January 2021 (0.44 ± 0.01) (One-way ANOVA, F2,11 = 28.22, p < 0.001, Tukey Post Hoc comparison analysis) (Table 2). When testing for spatial variations, RGL values were found to be significantly different between sites. The RGL value at Dam Doi, Ca Mau was the highest (0.57 ± 0.009, F2,3 = 3.52, p = 0.015), and the lowest was found in Duyen Hai, Tra Vinh and Tran De, Soc Trang (0.53 ± 0.01).
| Category | Specimens | RGL (Mean ± SE) | t-test | |
|---|---|---|---|---|
| Sex | Female | 508 | 0.55 ± 0.006a | t = 0.45, p = 0.15 |
| Male | 523 | 0.54 ± 0.007a | ||
| Season | Dry | 408 | 0.47 ± 0.006a | t = −12.90, p < 0.01 |
| Wet | 623 | 0.59 ± 0.006b | ||
| Size | Immature | 377 | 0.51 ± 0.007a | t = −5.39, p < 0.01 |
| Mature | 654 | 0.56 ± 0.006b | ||
| RGL (Mean ± SE) | One way-ANOVA | |||
| Year, Month | 2020 April | 93 | 0.47 ± 0.01abc | F = 28.22, p < 0.01 |
| 2020 May | 78 | 0.45 ± 0.008ab | ||
| 2020 June | 116 | 0.52 ± 0.012cd | ||
| 2020 July | 110 | 0.61 ± 0.01e | ||
| 2020 August | 80 | 0.65 ± 0.016e | ||
| 2020 September | 98 | 0.61 ± 0.018e | ||
| 2020 October | 64 | 0.64 ± 0.02e | ||
| 2020 November | 73 | 0.63 ± 0.019e | ||
| 2020 December | 82 | 0.50 ± 0.017bcd | ||
| 2021 January | 81 | 0.44 ± 0.01a | ||
| 2021 February | 74 | 0.48 ± 0.02abcd | ||
| 2021 March | 82 | 0.54 ± 0.02de | ||
| Site | Duyen Hai, Tra Vinh | 239 | 0.53 ± 0.009a | F = 3.52, p = 0.015 |
| Tran De, Soc Trang | 260 | 0.53 ± 0.01a | ||
| Dong Hai, Bac Lieu | 303 | 0.55 ± 0.009ab | ||
| Dam Doi, Ca Mau | 229 | 0.57 ± 0.009b | ||
Note:
Different letters (a, b, c, d and e) showed variation of RGL in each category.
Although the RGL of Ps. chrysospilos varied ontogenetically, all values were RGL < 1, indicating that this species is primarily carnivorous (Al-Hussaini, 1947). The interaction between factors, as assessed using GLM, such as gender × size (F2,1 = 1.48, p = 0.23); gender × season (F2,1 = 0.78, p = 0.38); gender x site (F2,1 = 0.92, p = 0.43); size × season (F2,1 = 0.24, p = 0.63); size × site (F2,1 = 1.39, p = 0.25); gender × size × season (F2,2 = 1.94, p = 0.16); gen × size × site (F2,2 = 2.31, p = 0.08); gender × season × site (F2,2 = 0.48, p = 0.70); size × season × site (F2,2 = 0.89, p = 0.45); gender × size × season × site (F2,3 = 0.33, p = 0.81), did not exhibit significant impact to the RGL with the exception of interaction season × site (F2,1 = 3.93, p = 0.008).
Diet composition
Analyses of the digestive tracts of 546 individuals of Ps. chrysospilos revealed clear patterns of food preferences (see Table 3 and Fig. 2). The three most-dominant food items from the pooled data of all individuals were, in descending order of percentage biovolume—Acetes spp. (small shrimps, 26.8%), Uca spp. (fiddler crabs, 21.6%) and Dolichoderus sp. (ant, 19.7%). Other food items present, by percentage biovolume, were Mollusca (molluscs, 16.7%), Polychaeta (bristleworms, 3.4%) and Actinopterygii (ray-finned fishes, 0.7%). Although relatively high in biovolume (11.2%) and occurrence (26.0%), detritus was low in weight (6.6% of total weight).
Figure 2: The food composition of Periophthalmus chrysospilos.
(A) Acetes spp., (B) Uca spp., (C) Mollusca, (D) Detritus, (E) Actinopterygii, (F) Dolichoderus sp., and (G) Polychaeta.| Food Item | % Biovolume | % Weight | % Occurrence |
|---|---|---|---|
| Acetes spp. | 26.8 | 21.5 | 19.2 |
| Uca spp. | 21.6 | 24.3 | 13.7 |
| Dolichoderus sp. | 19.7 | 16.2 | 18.8 |
| Detritus | 11.2 | 6.6 | 26.0 |
| Mollusca | 16.7 | 18.6 | 13.8 |
| Polychaeta | 3.4 | 9.4 | 5.6 |
| Actinopterygii | 0.7 | 3.4 | 2.9 |
The variation of occurrences, weight and biovolume of food items when compared to the sex and size of the fishes, as well as the site and season of catches, are illustrated in Table 4. The diet composition of Ps. chrysospilos did not vary under season and size parameters (PERMANOVA, df = 1, Pseudo-Fseason = 1.64, p = 0.16; df = 1, Pseudo-Ffish size = 0.54, p = 0.75) but did vary under the sex and site parameters (df = 1, Pseudo-Fgender = 3.86, pgender = 0.003; df = 1, Pseudo-Fsampling sites = 3.80, psampling sites = 0.001). The only diet component that drove the variance between males and females was ‘Uca spp.’ (Mann-Whitney U, df = 1, U = -1.93, p = 0.05), with females consuming more of these crabs than their male counterparts (see Fig. 3). The variation observed between sites were driven by five of the diet components: Mollusca, Uca spp., Dolichoderus sp. and detritus (Kruskal Wallis H test, df = 3, χ2Mollusca = 16.98, pMollusca = 0.001; df = 3, χ2Uca spp. = 8.15, pUca spp. = 0.04; df = 3, χ2Dolichoderus = 12.01, pDolichoderus = 0.007; df = 3, χ2detritu = 65.17, p detritus < 0.001). The three other food items, Acetes spp., Polychaeta and Actinopterygii, were not significant contributors for the variation observed between sites (df = 3, χ2Acetes = 2.39, pPolychaeta = 0.50; df = 3, χ2Polychaeta = 7.51, pPolychaeta = 0.06; df = 3, χ2Actinopterygii = 3.63, pPolychaeta = 0.30).
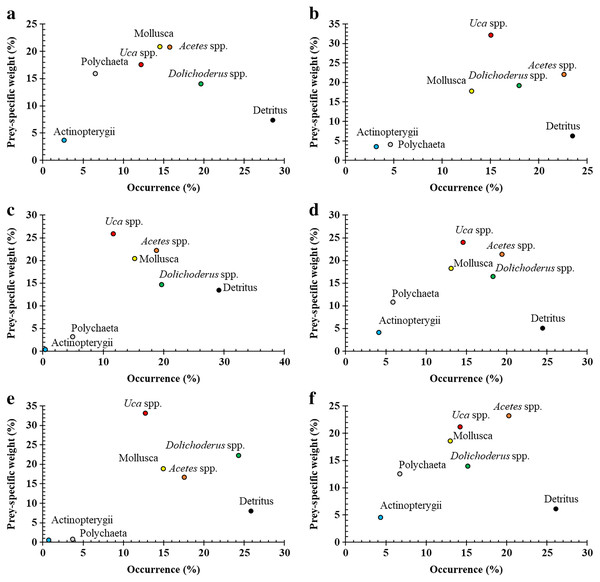
Figure 3: Modified Costello Graphs represent the feeding strategy of Periophthalmus chrysospilos based on the relationship between the percentage weight and percentage occurrence of food items between male and female individuals.
(A) male, n = 523; (B): female, n = 508); immature and mature individuals ((C): immature, n = 377; (D): mature, n = 654); and dry and wet seasons ((E): dry season, n = 408; (F): wet season, n = 623.| Parameter | Acetes spp. | Actinopterygii | Mollusca | Uca spp. | Polychaeta | Dolichoderus sp. | Detritus | PERMANOVA | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo-F | p | |||||||||
| % Occurrence | Female | 22.6 | 3.2 | 13.0 | 15.1 | 4.6 | 18.0 | 23.5 | 3.86 | 0.003 |
| Male | 15.8 | 2.7 | 14.6 | 12.2 | 6.6 | 19.6 | 28.6 | |||
| % Weight | Female | 22.1 | 3.2 | 16.7 | 30.2 | 3.8 | 18.0 | 5.9 | ||
| Male | 20.7 | 3.7 | 20.8 | 17.5 | 15.9 | 14.0 | 7.4 | |||
| % Biovolume | Female | 30.0 | 0.6 | 13.1 | 27.4 | 1.1 | 19.5 | 8.4 | ||
| Male | 22.7 | 0.7 | 21.0 | 14.8 | 7.2 | 19.0 | 14.6 | |||
| Mann-Whitney U test | U | −1.66 | −1.32 | −0.40 | −1.93 | −0.15 | −0.37 | −1.02 | ||
| p | 0.10 | 0.19 | 0.69 | 0.05 | 0.88 | 0.71 | 0.31 | |||
| % Occurrence | Dry | 17.6 | 0.8 | 15.0 | 12.7 | 3.8 | 24.3 | 25.8 | 1.64 | 0.162 |
| Wet | 20.3 | 4.4 | 13.0 | 14.3 | 6.8 | 15.2 | 26.1 | |||
| % Weight | Dry | 16.6 | 0.5 | 18.9 | 33.1 | 0.7 | 22.2 | 8.0 | ||
| Wet | 23.2 | 4.5 | 18.6 | 21.1 | 12.6 | 13.9 | 6.1 | |||
| % Biovolume | Dry | 16.7 | 0.0 | 16.2 | 24.1 | 0.2 | 31.0 | 11.9 | ||
| Wet | 31.6 | 1.3 | 16.2 | 20.2 | 5.7 | 14.2 | 10.7 | |||
| % Occurrence | Immature | 18.8 | 0.5 | 15.3 | 11.7 | 4.9 | 19.7 | 29.2 | 0.54 | 0.747 |
| Mature | 19.4 | 4.2 | 13.1 | 14.6 | 5.9 | 18.3 | 24.5 | |||
| % Weight | Immature | 22.2 | 0.3 | 20.4 | 25.9 | 3.2 | 14.6 | 13.5 | ||
| Mature | 21.3 | 4.1 | 18.3 | 24.0 | 10.8 | 16.5 | 5.1 | |||
| % Biovolume | Immature | 24.2 | 0.0 | 18.0 | 17.5 | 0.9 | 16.7 | 22.7 | ||
| Mature | 27.4 | 1.1 | 15.8 | 23.2 | 4.2 | 20.0 | 8.3 | |||
| % Occurrence | DHTV | 20.4 | 3.1 | 15.4 | 8.0 | 6.2 | 26.5 | 20.4 | 3.80 | 0.001 |
| TDST | 17.4 | 5.1 | 22.5 | 18.0 | 9.6 | 17.4 | 10.1 | |||
| DHBL | 18.9 | 2.4 | 10.1 | 12.4 | 3.6 | 18.9 | 33.7 | |||
| DDCM | 20.4 | 1.2 | 7.0 | 15.7 | 2.9 | 12.8 | 40.1 | |||
| % Weight | DHTV | 19.4 | 3.3 | 16.2 | 16.0 | 0.7 | 36.0 | 8.5 | ||
| TDST | 18.4 | 3.6 | 25.6 | 18.7 | 19.7 | 12.4 | 1.6 | |||
| DHBL | 25.5 | 2.8 | 16.6 | 24.8 | 3.8 | 18.4 | 8.2 | |||
| DDCM | 23.1 | 4.1 | 10.5 | 40.1 | 4.6 | 5.3 | 12.3 | |||
| % Biovolume | DHTV | 20.6 | 0.5 | 13.0 | 6.7 | 0.2 | 49.8 | 9.1 | ||
| TDST | 19.2 | 1.1 | 34.4 | 20.1 | 11.3 | 13.0 | 1.0 | |||
| DHBL | 30.2 | 0.4 | 10.5 | 19.2 | 0.8 | 21.7 | 17.2 | |||
| DDCM | 26.9 | 0.3 | 4.2 | 35.9 | 0.8 | 3.8 | 28.1 | |||
| Kruskal Wallis H test | χ2 | 2.39 | 3.63 | 16.98 | 8.15 | 7.51 | 12.01 | 65.17 | ||
| p | 0.50 | 0.30 | 0.00 | 0.04 | 0.06 | 0.01 | <0.01 | |||
Visualisations on the Costello graph (see Fig. 3) revealed Acetes spp. and Uca spp. to be the most significant prey items for Ps. chrysospilos, followed by Dolichoderus sp. and Mollusca with equal significance. Detritus, also high in occurrence, was not considered significant based on the position of this diet component within the lower right quadrant of the Costello graph. Both Polychaeta and Actinopterygii were considered less important prey items reflected by their positions within the lower left quadrant of the Costello graph. The diet composition of individuals of Ps. chrysospilos at the four sites (see Fig. 4) were significantly different. At DHTV, Dolichoderus sp. was the most important food item; at TDST, Mollusca was the item with the most significant contribution. At DHBL, Acetes spp. accounted for 30.2% biovolume, and was also by far, the most important prey item to Ps. chrysospilos here. At DDCM, Uca spp. was the main prey item and accounted for 35.9% of the biovolume of the mudskippers occurring at this site.
Figure 4: Modified Costello Graphs represent the feeding strategy of Periophthalmus chrysospilos based on the percentage weight and percentage occurrence of food items at four sampling sites.
(A) Duyen Hai, Tra Vinh, n = 239; (B) Tran De, Soc Trang, n = 260; (C) Dong Hai, Bac Lieu, n = 303; (D) Dam Doi, Ca Mau, n = 229.Discussion
Periophthalmus chrysospilos is an opportunistic carnivore based on its relative gut length and the contents within the digestive tracts of specimens examined. The RGL of this species fluctuated between values of 0.44 and 0.65, and falls within the range of a carnivorous fish with moderate feeding intensity (Al-Hussaini, 1947). These values corroborate those previously obtained for other Periophthalmus species (Clayton, 1993). An ontogenetic shift in the relative gut length was observed in this species, with larger specimens having longer digestive tracts. Seasonal variations in the RGL values could be attributed to the abundant available nutrient for rapid fish development during the wet season (July to November) (Hortle, 2009). The highest RGL values were disproportionately recorded from specimens collected at DDCM, an area with the highest flora diversity amongst sites. The variation in RGL values between sites was also reported in other confamilial taxa—Periophthalmodon septemradiatus (Dinh, Tran & Nguyen, 2018) and Butis koilomatodon (Nguyen, Lam & Dinh, 2020)—from the Mekong Delta. The varied diet composition of this amphibious fish species reflects the habitat in which it resides and the interactions between marine and terrestrial biota within the intertidal areas. The dominant diet component of this species is Acetes spp, small marine shrimps that are not tolerant of emersion out of water. The second-most dominant component of its diet is the fiddler crabs, Uca spp, that are active on exposed sand or mudflats during the ebb tide. Next in dominance by diet composition is Dolichoderus sp., terrestrial ants that are found at or near mangrove forests.
Within the Mekong Delta, Acetes spp., Uca spp., Dolichoderus sp. and many molluscs species are commonly available (Cuc & Van Mele, 1999; Dinh et al., 2020b). Their availability at the sites determines their dominance in the diet composition of this mudskipper species. For example, Dolichoderus sp. was the most important prey item for specimens collected at DHTV, while Acetes spp. was the main food item for specimens from DHBL. Unsurprisingly, the highest diet components of Ps. chrysospilos from elsewhere differ slightly to those from our study; specimens of this species from southern Sumatra predominantly consumed Uca sp. and fish eggs (Ridho, Patriono & Solikha, 2019). Similar to our study, detritus accounted for a significant proportion of the diet composition. However, as with our study, polychaete worms and small fishes were rarely encountered as diet components of specimens from Sumatra, Indonesia (Ridho, Patriono & Solikha, 2019) and Tanjung Piai, Peninsular Malaysia (Hui et al., 2019). The tolerance to a wide diversity of food items suggests the adaptability of this species to constant environmental changes within the intertidal habitats.
The diet composition of Ps. chrysospilos was not affected by season and fish size but differed according to sex and sites. Females consumed more Uca spp. than males. Gender-associated feeding modes was also observed in the congener Ps. barbarus (Linnaeus, 1766)—more cyanobacteria, Coscinodiscus spp. and Sesarma spp. were found in the digestive tracts of males than females (Udo, 2002b). This differential preference in food intake was also observed in a closely-related genus Periophthalmodon (hereafter ‘Pn’.), in which the males of Pn. schlosseri typically consumed Uca spp. while females exhibited preference for the ricefish Oryzias sp. (Zulkifli et al., 2012). The site, and by extension the food availability therein, was a driver for the differences in the main food item ingested by Ps. chrysospilos —Dolichoderus sp., Mollusca, Acetes spp. and Uca spp. were the principal prey item for specimens collected from DHTV, TDST, DHBL and DDCM respectively. Similarly, the diet of congener Ps. argentilineatus from Zanzibar consisted predominantly of amphipods and copepods, whereas in mainland Tanzania, Polychaeta was the main food source (Kruitwagen et al., 2007).
There appear to be no changes in food preferences between Ps. chrysospilos of size ranges from 3.4 cm to 10.6 cm in this study, an observation contrary to mudskippers of other species. Periopththalmus argentilineatus collected from Iriomote Island (Japan) showed a significant shift in dietary preference from Polychaetae in smaller individuals to Uca spp. for larger individuals (Nanjo, Kohno & Sano, 2008). The contribution of Uca spp. is more significant in larger and mature specimens of Pn. schlosseri (Zulkifli et al., 2012) and Parapocryptes serperaster (Dinh et al., 2017); the dietary composition of smaller individuals of the latter species consisted mostly of Dolichoderus sp. (Dinh et al., 2017). The dietary composition of Ps. chrysospilos is independent of seasons, unlike its congener Ps. barbarus (observed in the Niger Delta) that exhibited a shift to the significantly higher consumption level of three food items, namely unidentified crab parts, Penaeus sp, as well as annelid and nematode worms in the wet season compared to the dry season (Chukwu & Deekae, 2013). Analyses of digestive tracts also indicate that detritus and organic matter are ingested incidentally by this species. Despite the increased availability of these two items during the wet season (Nedeco, 1993), their composition within the diet of Ps. chrysospilos remained similar between seasons The dominance of detritus to dietary composition in Ps. barbarus (from Nigeria) however, persisted only during the wet season but changed to algae-dominant in the wet season (Udo, 2002a).
In conclusion, Periophthalmus chrysospilos is demonstrated to be an opportunistic mesopredator, and a carnivore with moderate feeding intensity, as evidenced by the RGL value (<1) and an equal ratio of fishes with empty and full digestive tracts. The food composition of Ps. chrysospilos consisted of seven types of food items: Acetes spp., Uca spp., Dolichoderus sp., Mollusca, Polychaeta, Actinopterygii, and detritus. The principal food item of this mudskipper species varied depending on site and food availability, thus indicating its adaptability in pursuing prey items within the limits of littoral zone. Exploited organisms range from marine shrimps to intertidal crabs and terrestrial ants, signalling the importance of this amphibious species as a conduit of terrestrial-marine connectivity within littoral landscapes.